Blue hydrogen production: process & considerations
As infrastructure expands, blue hydrogen from steam methane reforming (SMR) or autothermal reforming (ATR) with CCUS balances cost and environmental goals, driving research, technological innovation and growth in the hydrogen energy sector

In brief
- Blue hydrogen refers to hydrogen produced from natural gas with carbon capture, utilization and storage (CCUS). The color blue was adopted to signal a lower-carbon evolution of grey hydrogen.
- The two primary methods for producing blue hydrogen are steam methane reforming (SMR) and autothermal reforming (ATR). ATR provides higher carbon capture efficiency but requires a higher upfront investment.
- CCUS is integrated into blue hydrogen production to limit CO₂ emissions to the atmosphere. While current CCUS implementations remain cost-intensive, ongoing advances in capture efficiency, integration, and storage performance offer clear opportunities for optimization.
- While long-term sustainability considerations remain, blue hydrogen provides superior near-term economic and system viability relative to green hydrogen and improved emissions performance over grey hydrogen through CCUS integration, supporting its role as a transition fuel.
- Efficient SMR and ATR processes depend on the proper instrumentation to ensure efficiency, safety and optimal hydrogen purity.
Meeting current hydrogen demand
As climate change and global carbon reduction goals spur hydrogen energy exploration, many hydrogen production methods are emerging, each with distinct benefits and challenges. While green hydrogen - produced entirely from renewable sources - embodies the ideal of a sustainable future, its current economic, technological and scalability limitations require significant augmentation by other colors of hydrogen production to continue propelling this fuel’s viability.
Grey and blue hydrogen currently comprise the majority of hydrogen produced globally, both created via readily accessible steam methane reforming (SMR) or autothermal reforming (ATR), typically leveraging natural gas as a feedstock. While both hydrogen colors rely on these same production methodologies, the blue hydrogen process goes one step further than the grey one by capturing and storing the carbon emissions generated alongside produced hydrogen to prevent the former’s release into the atmosphere. For this reason, it is considered low-carbon hydrogen.
Insights
Grey and blue hydrogen currently comprise the majority of hydrogen globally, produced via readily accessible steam or autothermal reforming of methane, typically leveraging natural gas as a feedstock.
Blue hydrogen production methods
Steam methane reforming (SMR)
Steam methane reforming (SMR) is a mature thermochemical process, in which a methane source, such as natural gas, is reacted with high-temperature steam at 3-25 bar (43.5-363 psi) in the presence of a catalyst. It has a long history in industries like refining, fertilizer manufacturing and methanol production.
This reaction yields syngas, a mixture of hydrogen and carbon monoxide. A subsequent water-gas shift (WGS) reaction then converts the carbon monoxide to additional hydrogen, generating carbon dioxide and a small amount of carbon monoxide as byproducts.
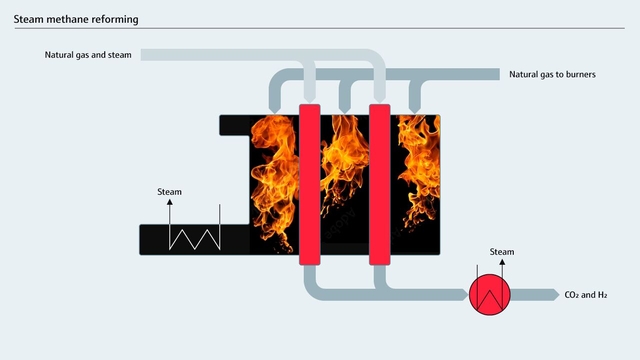
Steam methane reforming in the reformer unit
How steam methane reforming works: the chemical process
Blue hydrogen SMR requires three key reactions and an optional fourth step.
1. Methane reforming
In this primary reaction, methane (CH4) - typically from natural gas - is reacted with steam (H2O) at high temperature (700-1,100 °C/1,300-2,000 °F) and pressure (3-25 bar/43.5-363 psi) in the presence of a nickel-based catalyst. This reaction produces syngas, a mixture of hydrogen (H2) and carbon monoxide (CO). It is endothermic, therefore requiring heat input.
CH4 + H2O ⇌ CO + 3H2 (ΔH = +206 kJ/mol)
2. Water-gas shift reaction
The syngas then undergoes a water-gas shift reaction (WGS reaction), whereby carbon monoxide reacts further with steam in the presence of a catalyst, usually iron oxide or copper-based, to produce more hydrogen and carbon dioxide (CO2). This reaction is exothermic, meaning it releases heat.
CO + H2O ⇌ CO2 + H2 (ΔH = -41 kJ/mol)
3. Carbon dioxide removal
The resulting gas mixture at this stage is primarily comprised of hydrogen, carbon dioxide and some unreacted methane. Carbon dioxide is most removed via amine gas treatment, which entails dissolving carbon dioxide in amine solutions, leaving behind a purified hydrogen stream.
CO2 + Amine Solution ⇌ Amine-CO2 Complex (simplified chemical representation)
4. Hydrogen purification (optional)
Depending on the desired purity level, further purification steps may be employed. Pressure swing adsorption (PSA) - which uses adsorbent materials to selectively capture carbon dioxide - and membrane separation, which employs specialized membranes allowing only hydrogen to pass through, are the two most common methods.
Catalysts are essential to SMR to speed up reactions, but they become depleted over time and must be regenerated or replaced. The endothermic methane reforming process and the exothermic WGS reaction require careful heat management for efficient operation.
Autothermal reforming (ATR)
Autothermal reforming (ATR) is a newer method, particularly well-suited for large-scale hydrogen production. While the equipment to induce the required reactions requires greater capital investment, this method promotes more efficient carbon capture. This is a result of controlled oxygen gas dosing in the reformer unit, which reduces carbon monoxide output, thus producing a purer stream of carbon dioxide than SMR.

Autothermal reforming in the reformer unit
Additionally, because ATR partially oxidizes methane with oxygen to produce syngas, it does not require an external heat source. As with SMR, a water-gas shift reaction maximizes the hydrogen yield.
How autothermal reaction (ATR) works: the chemical process
1. Feedstock preheating and mixing
Natural gas - primarily methane - and steam are preheated and controlled amounts of oxygen (O2) are added to the mixture.
2. Combustion
A portion of the methane reacts with the added oxygen in a highly exothermic combustion reaction, generating heat for the subsequent reforming reaction.
CH4 + 2O2 → CO2 + 2H2O (ΔH = -890 kJ/mol)
3. Reforming
The heat generated during combustion drives the endothermic reforming reactions.
Steam reforming: CH4 + H2O ⇌ CO + 3H2 (ΔH = +206 kJ/mol) Partial oxidation: 2CH4 + O2 ⇌ 2CO + 4H2 (ΔH = -36 kJ/mol)
4. Water-gas shift reaction
Like SMR, the carbon monoxide produced in the reforming reactions reacts further with steam in the presence of a catalyst to produce more hydrogen and carbon dioxide:
CO + H2O ⇌ CO2 + H2 (ΔH = -41 kJ/mol)
5. Carbon dioxide removal
Like in SMR, carbon dioxide is removed from the gas mixture, most frequently using amine gas treatment, which leaves behind a purified hydrogen stream.
6. Hydrogen purification (optional)
Further purification steps, such as PSA or membrane separation, can be implemented to achieve increased hydrogen purity when required.
SMR is simpler and less expensive to implement than ATR because the former does not require a constant oxygen source. However, ATR is self-sustaining in terms of heat due to the integrated combustion reaction, so it does not need an external heat source once it is going, making it more energy-efficient than SMR.
Additionally, ATR typically produces a higher ratio of hydrogen to carbon monoxide in the syngas, which can be advantageous for some downstream applications. ATR systems are also usually capable of faster responses to changes in production demands. For these and other reasons, new blue hydrogen facilities typically use ATR.
Considering SMR versus ATR
The decision of whether to pursue steam methane reforming or autothermal reforming for blue hydrogen production boils down to a comprehensive assessment of several factors, including but not limited to:
- Desired scale of production
- Required hydrogen purity
- Composition of the available natural gas feedstock
- Access to capital
- Projected operational costs
- Global or regional economic landscape
The importance of carbon capture and storage in blue hydrogen production
A discussion of blue hydrogen is incomplete without covering carbon capture, utilization and storage (CCUS). These complex processes begin with separating carbon dioxide from other gases present in an exhaust stream, which often relies on absorption-based technologies using e.g. amines that selectively capture carbon.
Once captured, the carbon dioxide undergoes compression and liquefaction to a supercritical state, enabling efficient transportation - typically via pipeline - to suitable geological formations for long-term storage. Potential storage sites include depleted oil and gas reservoirs, deep saline aquifers and salt domes.
While carbon capture and storage (CCS) sites offer a way to manage emissions, there are some questions about their long-term security. Even small leaks could potentially affect nearby ecosystems and groundwater.
Blue hydrogen considerations
There’s ongoing discussion about the environmental impact of blue hydrogen compared to green hydrogen, which is produced using renewable energy. Some suggest that focusing on blue hydrogen might delay the transition to renewable energy and green hydrogen.
From a financial perspective, the costs associated with CCS can make blue hydrogen technology more expensive than grey hydrogen. However, these costs are gradually decreasing. Additionally, factors like carbon taxes on grey hydrogen, government incentives for blue hydrogen and cap-and-trade systems could make blue - or even green - hydrogen more economically viable.
Advantages of blue hydrogen
With the expansion of hydrogen infrastructure, blue hydrogen plays a critical transition role by supporting near-term deployment, efficiency gains and continued innovation as green hydrogen matures. SMR and ATR remain the most commercially viable production pathways, balancing cost, emissions reduction and technological readiness. Ongoing advances in CCUS are improving capture performance and storage integrity, though significant investment and scale are still required for blue hydrogen to materially displace grey production.
As the global energy picture evolves, progress will rely on a pragmatic, technology-inclusive strategy that recognizes trade-offs among hydrogen variants and prioritizes scalable, long-term solutions. The energy transition will require multiple hydrogen colors, renewable energy sources, expanded electrification and even efficient use of fossil resources with emissions mitigation. Deploying the right mix of solutions for each application will be essential to delivering a reliable and competitive low-carbon energy system.
Instrumentation’s role for blue H2
Beyond environmental and economic considerations, the successful deployment of blue hydrogen production hinges on a sophisticated network of instrumentation and control systems working in concert to ensure process reliability, efficiency and safety. SMR and ATR require vast arrays of sensors to constantly monitor process parameters and feed real-time data into elaborate control systems to optimize production, minimize waste and mitigate risks.
Insights
The successful deployment of blue hydrogen production depends on a sophisticated network of instrumentation and control systems working in concert to ensure process reliability, efficiency and safety.
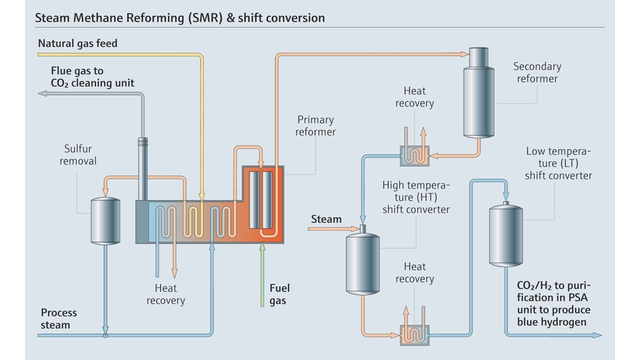
Temperature sensors, crucial for maintaining optimal reaction conditions and preventing catalyst degradation, operate in tandem with pressure sensors that ensure safe conditions within reactors and pipelines. Flowmeters reliably document the movement of gases and liquids throughout the process, enabling precise control of reactant ratios and product streams. Flowmeters are also critical at all points of custody transfer.
Meanwhile, gas analyzers - such as Raman analyzers and tunable diode laser absorption spectroscopy (TDLAS) - provide stream composition and other monitoring at various points, empowering operators to validate process efficiency, detect issues as they develop and ensure hydrogen purity.




